

Electron Dot Structures
Introduction
Electron dot structures are a visual representation the valence electrons around an atom or bond.
What Are Those Dots?
The dots in an electron dot diagram represent an atom's valence elections, which occupy the outermost energy level and are involved in chemical reactions. Dot diagrams may be used to describe single atoms as well as covalent and ionic bonds.
Single Atoms
Single atoms have a specific number of valence elections depending on their period. All the elements in the same period have the same number of valence electrons. For example, all the elements in period 1 have 1 valence electron. Therefore, hydrogen, lithium, sodium, potassium, etc. all have 1 valence electron. All the elements in period 6, including oxygen, sulfur, and selenium, have 6 valence electrons.
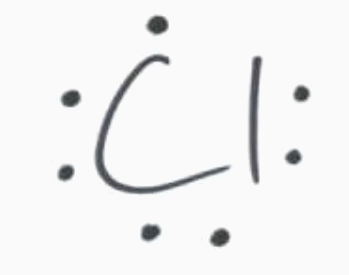
If an element has 1 valence electron, like potassium, then the electron dot diagram will include one dot. A noble gas like neon has a full outer shell, or 8, electrons. And chlorine has 7 dots representing its 7 valence electrons.
| Potassium | Neon | Chlorine |
|
|
|
|
Now try drawing your own electron dot diagram for the following elements:
sodium, lithium, argon, iodine, magnesium, boron
Covalent Bonds
Covalent bonds share electrons. Covalent bonds take place between two nonmetals and are generally weaker than ionic bonds. Here is an example of how to draw an electron dot structure for a single bonded water molecule.
Single Bonds:


Now try drawing the electron dot structure for the following molecules:
CH4, F2, and H2S
Double Bonds:




Now try these:
O2, CH2O, and C2H6
Triple Bonds:




Now try:
C2H2